Abstract
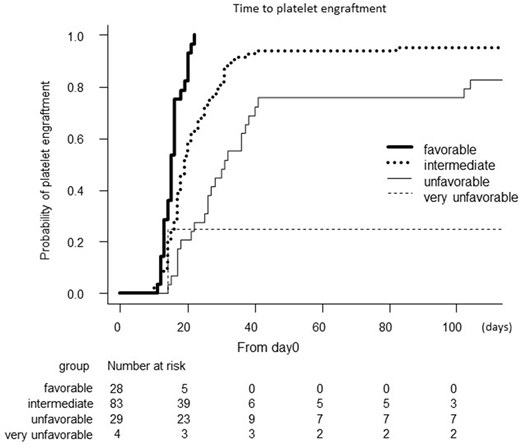
Transient thrombocytopenia is common after autologous peripheral blood stem cell transplantation (auto-PBSCT). Although patients underwent auto-PBSCT usually show the recovery of peripheral leukocytes and platelets counts within a couple of weeks after auto-PBSCT, delayed platelet engraftment (DPE) is occasionally observed despite prompt neutrophil engraftment. However, there are few studies evaluating the risk factors for DPE. In this study, to investigate the association between clinical characteristics and duration required for post-auto-PBSCT platelet engraftment, we retrospectively analyzed 144 patients with B-cell non-Hodgkin lymphomas who underwent auto-PBSCT between January 2000 and December 2017 at 5 independent institutes involved in the Kyoto Clinical Hematology Study Group. Platelet recovery was defined as a recovery of platelet count greater than 50.0 × 109/L without transfusion support for 3 consecutive days, and the first day of 3 consecutive days was regarded as the day of platelet engraftment. The median observation period was 930 days (range, 25 - 5272 days). Median age at auto-PBSCT was 61, and 144 patients included 109 diffuse large B cell lymphoma, 11 follicular lymphoma, 22 mantle cell lymphoma. Ninety one patients were in complete remission at the time of auto-PBSCT. The mobilization regimens for stem cell collection were chemotherapy such as high-dose VP16 (46.5%), CHASE (cyclophosphamide, AraC, VP16, and dexamethasone) (40.3%), and high-dose AraC (8.3%) followed by G-CSF. All 144 patients except one achieved neutrophil engraftment with the median engraftment day of 10 days (range, 4-41 days), and 139 patients achieved platelet engraftment with the median engraftment day of 19 days (range, 10-1125 days). Multivariate analysis showed that infused CD34-positive cell number (≥ 2.0 × 106/kg vs. < 2.0 ×106/kg: HR 3.06: P < 0.001), pre-apheresis absolute lymphocyte count (PA-ALC), which was the mean number of lymphocytes during aphaeresis procedure (≥ 1.0 × 109/L vs. < 1.0 × 109/L: HR 2.62: P < 0.001), and the days required for apheresis procedure (≥ 3 days vs. 1 or 2 days: HR 0.49: P = 0.02) were independently associated with the duration for platelet engraftment after auto-PBSCT. Age, gender, diagnosis, Ann Arbor stages, previous history of bone marrow infiltration, performance status, international prognostic index (IPI)-defined disease risk, disease status at the time of transplant, the number of chemotherapy regimens, and mobilization regimen were not associated with platelet engraftment period. On the basis of these results, we next tried to create a new prediction system for post-transplant platelet recovery by incorporating the three factors, i.e., infused CD34-positive cell number, PA-ALC, and days required for apheresis procedure. Risk groups were determined by the sum of predictive factors for platelet recovery: favorable (3 factors), intermediate (2 factors), unfavorable (1 factor), and very unfavorable (no factor). As the results, the median duration for platelet engraftment with favorable, intermediate, unfavorable, and very unfavorable groups were 15, 19, 31, and 148 days, respectively (P < 0.001) (Figure). Furthermore, 14 of 144 patients did not achieve platelet engraftment by day 80 after auto-PBSCT. We defined those as patients with DPE. Median duration for platelet engraftment in patients with DPE was 137 days (range, 82-1125 days), while that for neutrophil engraftment was 11 days. Intriguingly, PA-ALC was less than 1.0 × 109/L in all 14 patients with DPE, while 6 required apheresis for ≥3 days, and 5 received <2.0 ×106/kg CD34-positive cells for transplantation. Collectively, the newly developed scoring system which incorporates infused CD34+ cell dose ≥ 2.0 × 106/kg, PA-ALC ≥ 1.0 × 109/L, and number of apheresis procedures ≤2 days, is an useful tool for predicting platelet recovery after auto-PBSCT. Especially, patients with low PA-ALC are high risk of DPE after auto-PBSCT. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki, and was approved by the Institutional Review Board of our institutes.
Kuroda:Chugai Pharma: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal